Veeva is the global leader in cloud software for the life sciences industry, serving more than 1,100 customers, ranging from the world’s largest pharmaceutical companies to emerging sites. As a Public Benefit Corporation, Veeva is committed to balancing the interests of its customers, employees, shareholders, and the industries it serves. Learn more at veeva.com.
Veeva SiteVault is the only unified solution connecting sites with sponsors and patients to run digital trials. Streamline regulatory processes and connect to 400+ sponsors that use Veeva Clinical applications. Learn more at sites.veeva.com.
Products
Veeva SiteVault
Veeva SiteVault Free is a 21 CFR Part 11 and HIPAA compliant eRegulatory application used by more than 4,000 clinical research sites across over 75 countries. SiteVault Free replaces paper binders and manual processes and connects sites to over 400 sponsors using Veeva Clinical applications.
Videos
Veeva SiteVault Free – What is it?
Mid Hudson Medical Research Improves Clinical Trials with SiteVault
Images
Documents
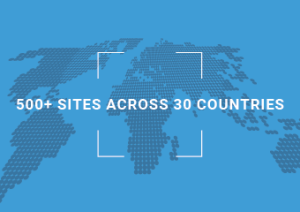
More Than 500 Clinical Research Sites Adopt Veeva SiteVault to Accelerate Research
In eight months since the product has been available from Veeva Systems (NYSE: VEEV), the number of SiteVault Free customers has increased to more than 500 in over 30 countries, signaling a rapid shift among global research sites to simplify and streamline study execution.
Tilda Research Speeds Study Activation and Cuts Administrative Work in Half with Veeva SiteVault
Faced with mountains of paper documents, labor-intensive processes, and rising operational costs, a large site network turns to digital solutions to speed study execution across all of their studies.
Going Digital with Remote Monitoring: Key Considerations
With many monitors now restricted from travel, sites and sponsors are reassessing ways to share information and collaborate virtually. Explore best practices for getting started with remote monitoring.
Veeva Announces Free eRegulatory Solution for Clinical Research Sites
PLEASANTON, CA — Oct. 3, 2019 — Veeva Systems (NYSE:VEEV) today announced Veeva SiteVault Free, a free eRegulatory solution built specifically for clinical research sites.